FDA Breakthrough Device Designation for Third-party Interoperability
by Saira Khan-Gallo, representative of the nonprofit Tidepool
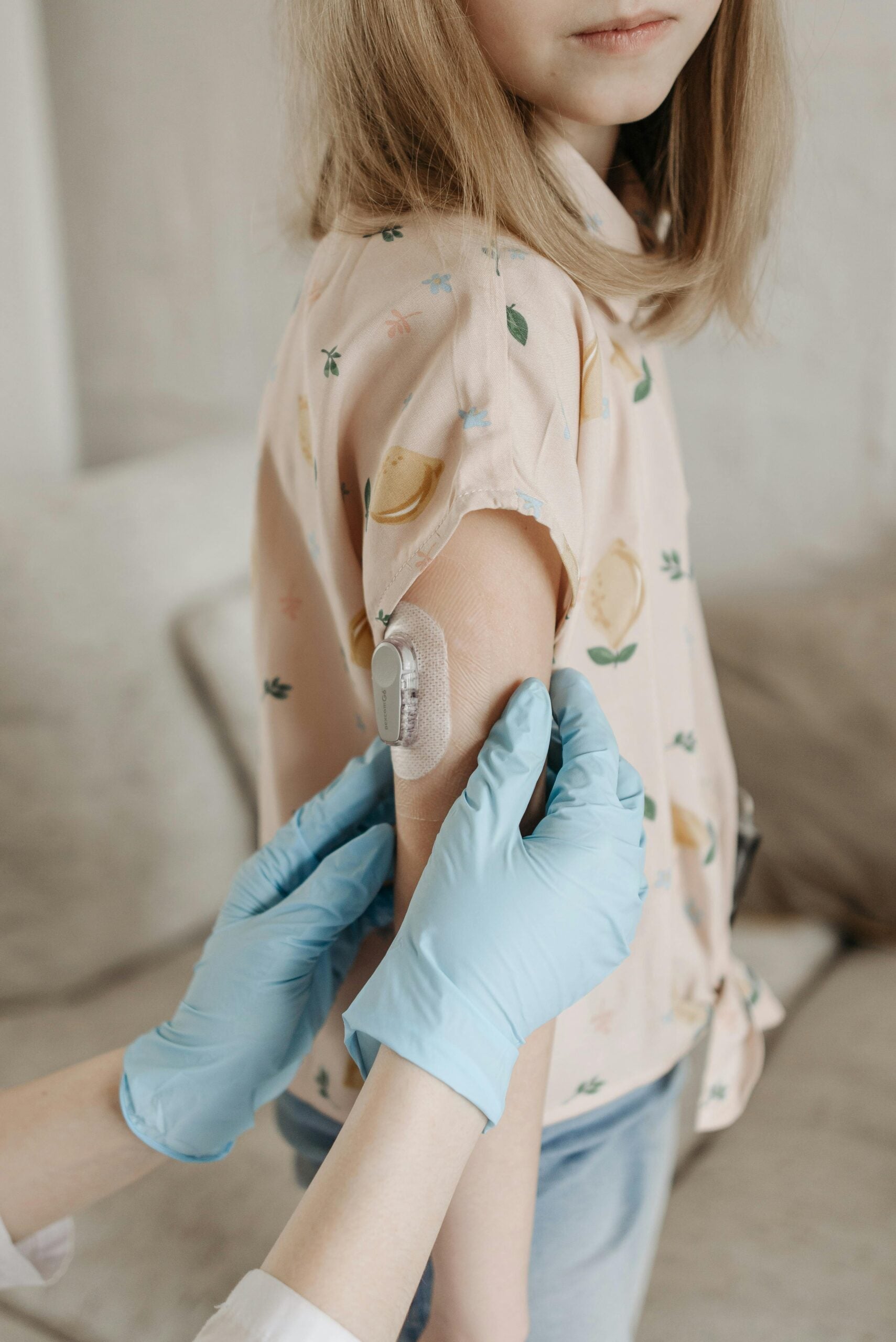
Interoperability between devices from different manufacturers can improve patient care for people with diabetes. This project recommends that the Food and Drug Administration (FDA) should make it explicit that third-party interoperability for medical devices is a qualifier for expedited medical device approval through its Breakthrough Device Designation program. By making it clear to medical device sponsors that offering a third-party interoperable system would qualify their device for the Breakthrough Device Designation, the FDA will incentivize sponsors to consider third-party interoperability – enabling patient choice and faster innovation in the medical device industry.
This project was completed as part of the 2023 Summer Nonprofit and Public Interest Fellowship, a Hub program to support public interest organizations in achieving policy impact. Project author Saira Khan-Gallo served as a representative of the nonprofit Tidepool, a nonprofit that aims to make diabetes data more accessible, actionable, and meaningful.




 Trust? Verify! Dealing with Social Media Deception
Trust? Verify! Dealing with Social Media Deception